Thyroid fine-needle aspiration biopsy
A specific appointment is scheduled to perform the procedure, whose duration varies but generally ranges from 10 to 15 minutes, depending on the technical difficulty and number of biopsies needed.
The patient must sign a consent form prior to the procedure.
The patient is lying down in supine position. The specimen is removed under sterile conditions and ultrasound guidance, using very fine needles (25 to 27 gauge). One to three samples will be removed and placed on a slide or in a liquid-based solution. The practitioner can see the tip of the needle at all times during the procedure. Complications are rare (generally minimal hematoma at the insertion site).
The specimens are sent to the laboratory for cytologic analysis. Results are available within five to ten days.
The cytologist uses the 2023 Bethesda System:
- Bethesda I: nondiagnostic sample (cell specimen insufficient or containing too much blood)
- Bethesda II: benign
- Bethesda III: atypia of undetermined significance
- Bethesda IV: follicular nodule/suspicious follicular nodule
- Bethesda V: suspicious for malignancy
- Bethesda VI: malignant
The results are sent to the prescribing physician and given to the patient during a specific disclosure consultation.
We also perform FNA biopsy on lymph nodes in the neck and on other neck lumps.
HOW IS THYROID FNA BIOPSY PERFORMED?
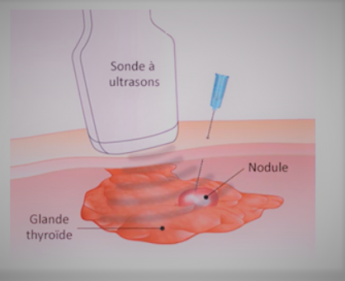
*Nodule
*Thyroid gland
2023 Bethesda System for Reporting Cervical Cytology
The 2023 Bethesda System for thyroid nodules is an international classification that enables cytopathologists to score thyroid nodules after a FNA procedure based on the risk, thereby helping clinicians more efficiently manage the disease while ensuring patients benefit from complete information transparency.
This third version of the Bethesda System assigns a single name to each of the six diagnostic categories for thyroid nodules: (i) Nondiagnostic; (ii) Benign; (iii) Atypia of undetermined significance; (iv) Follicular nodule; (v) Suspicious for malignancy; (vi) Malignant. Each of the six categories has an implied risk of malignancy (ROM), which has been updated and refined based on data reported after the second edition of the Bethesda System. The third edition offers an average ROM for each category, in addition to the expected range of cancer risk. The atypia of undetermined significance subcategorization is simplified into 2 subgroups based on the implied ROM and the possible use of molecular profiling.
The nomenclature has been updated to align with the 2022 World Health Organization classification of thyroid tumors. Two new chapters have been added: one that that summarizes clinical perspectives and imaging findings in thyroid disease, and another that addresses the significant and expanded use of molecular testing, which is still too costly to be covered by the relevant health care systems.
The 2023 Bethesda System for Reporting Thyroid Cytopathology
| Bethesda category | Diagnostic category | Average risk of malignancy in % (upper and lower thresholds in parentheses) | Usual management |
| 1 | Nondiagnostic | 13 (5-20) | Repeat FNA under ultrasound guidance |
| 2 | Benign | 4 (2-7) | Clinical and sonographic follow-up |
| 3 | Atypia of undetermined significance | 22 (13-30) | Repeat FNA, molecular testing, or lobectomy or surveillance |
| 4 | Follicular nodule | 30 (23-34) | Molecular testing or lobectomy |
| 5 | Suspicious for malignancy | 74 (67-83) | Molecular testing or near-total thyroidectomy or lobectomy |
| 6 | Malignant | 97 (97-100) | Near-total thyroidectomy or lobectomy |